
A comparison of the UV-vis absorption spectra from density functional theory calculations and experiment showed how a p-conjugated system's HOMO-LUMO gap was tunable with structural modifications to the core, which illustrated how physical organic principles could be used to understand the electronic properties of a p-conjugated system. The students characterized their products with TLC, NMR, IR, and UV-vis (from a representative sample) spectroscopies. The synthesis of electron-rich and electron-poor biphenyl compounds was accomplished by students with a modified procedure from the literature that uses green Suzuki conditions to yield 4'-("tert"-butyl)-4-ol (1) and methyl 4'-("tert"-butyl)-4-carboxylate (2) with average yields of ~50% and ~60%, respectively.

In order to demonstrate a general strategy for tuning the HOMO-LUMO energy gap for p-conjugated materials, a new experiment was designed for the undergraduate organic laboratory course that combines synthesis with theoretical calculations for p-conjugated biphenyl systems with differing electronic properties to generate a simple structure-property study of functional group substitution on the biphenyl core.
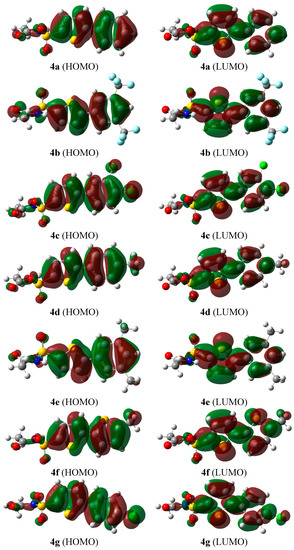
For instance, the ability to modify the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) in p-conjugated small molecules and polymers is of particular interest in the fields of polymer chemistry and materials science, where small-gap systems can function as semiconductors in electronic devices. HOMO HOMO If two HOMO orbitals react then two new MO’s will be obtained (one lower and one higher in energy) The 4 electrons will be placed and thus there is no energy gain LUMO LUMO If two LUMO orbitals react then also obtain two new MO’s, but since there are no electrons, there is no change in energy HOMO LUMO The only energy. Preliminary results on OPV device preparation and characterization are also discussed.While structure-property relationships are commonly developed in applications of physical organic chemistry to real-world problems at the graduate level, they have not been generally emphasized in the undergraduate chemistry curriculum. According to Xavier 16, are said frontier molecular orbitals those where the chemical reactions actually occur. The effect is so strong that the derivative bearing the strongest acceptor no longer behaves as a donor semiconductor. The low energy shift effect on the HOMO level with respect to the parent unsubstituted squaraine can be as high as 0.3 eV. Our data enable the establishment of interesting structure–property relationships relating the nature, position and number of electron-withdrawing substituents to the position of the donor HOMO and lowest unocupied molecular orbital (LUMO) levels. These new semiconductors have been characterized by optical spectroscopy (UV/Vis absorption and IR transmittance) and electrochemical measurements (cyclic voltammetry and differential pulsed voltammetry).
#HOMO AND LUMO ORGANIC CHEMISTRY SERIES#
In this study a series of arylhydrazone end-capped symmetric squaraine compounds bearing electron-withdrawing substituents on the aryl ring has been synthesized with the aim of increasing the device open circuit voltage by shifting the highest occupied molecular orbital (HOMO) energy level. Squaraine compounds represent one of the most promising small molecule materials to be employed as the donor in the bulk heterojunction (BHJ) OPV configuration. HOMO-LUMO gap: The energy difference between a molecule's Highest energy Occupied Molecular Orbital and its Lowest energy Unoccupied Molecular Orbital.

Design principles could be developed from these analyses, which led to a proof-of-concept linear D-A with a strong excited-state intramolecular charge transfer and a NIR absorption at 879. The optimization of organic photovoltaic (OPV) devices requires, amongst several factors mainly related to thin-film blend morphology, the capability to finely tune the characteristic energy levels of both the donor and the acceptor materials constituting the active layer. The trends in HOMO-LUMO gaps of the model dyes correlate with the excitation energies computed with time-dependent density functional theory at CAMY-B3LYP.


 0 kommentar(er)
0 kommentar(er)
